Our Keymical Collections™ provides novel, tractable, and inspired-by-nature chemical matter for your screening campaigns. You can choose original exclusive Compound Collections – Covalent, Fragment, and DEL libraries – for hit generation and we care about it for you with our Compound Management and Logistics.
Our EDEN (Edelris Discovery ENgine) propels the unique combination of Affinity Selection – Mass Spectrometry (AS-MS) screening and our multimillion compound proprietary Keymical Space ™ towards discovering novel hits for your targets.
Our expert and resourceful scientists in Organic, Medicinal, and Analytical chemistry enjoy the intellectual challenge and success in efficiently optimizing your active molecules on your behalf in Hit-To-Lead & Lead-Optimization.
Our DNA is about serving our clients with creativity & reliability. Let’s discover together tomorrow’s drugs.
Missions and values
We are a technology-driven CRO in early phases of Drug Discovery.
We aim to support drugging the undruggable, by developing a differentiated technology-platform able to efficiently identify targeted hits and optimize leads.
We bring to clients innovative solutions and premier and novel chemical matter to advance more efficiently their small molecules drug discovery programs.
We create original solutions to expand the boundaries of small molecules drug discovery.
Succeeding in our Missions is essential for us. The way we do it, as well. Here are the core values guiding our way, in our daily work as a team, and in our relationships with our clients and partners.
Creativity
- We imagine and offer innovative solutions to meet the needs of our customers.
- We explore and implement new paths.
- We cross-fertilize experiences and technologies to push the boundaries of discovery.
Adaptability
- We adapt to the needs of our clients and their strategies while integrating versatility.
- We challenge ourselves to improve and evolve.
- We stay agile to environmental changes and offer a flexible work environment.
Commitment
- We face challenges and win together.
- We are committed to giving our best to achieve our goals.
- We deliver on time and to the highest quality standards.
Teamwork
- We build trust-based relationships both internally and externally.
- We help each other, listen, respect, and share.
- We collaborate with our customers and partners to leverage the best solutions.
Excellence
- We provide the highest level of scientific expertise and interpersonal skills.
- We persevere to break through to tangible results for our customers.
- We solve complex challenges thanks to our cutting-edge science and smart solutions.
Leadership
Founders
Representative success stories
On behalf of Cedilla Therapeutics, we used the EDEN™ (Edelris Discovery ENgine) platform – a combination of our unique 2M+ natural product mimetic compound collection and Affinity Screening – Mass Spectrometry (AS-MS)1 technology to discover premium chemical binders.
Tapping into this unique collection revealed several biorelevant ligands, confirmed by Cedilla in stringent orthogonal screens, incl. X-Ray crystallography.
Based upon our extensive chemistry knowledge of the identified ligands, the hit exploration and hit-to-lead optimization is currently ongoing in our laboratories².
1 Exploring new targets and chemical space with affinity selection-mass spectrometry. Renaud Prudent, D. Allen Annis, Peter J. Dandliker, Jean-Yves Ortholand, Didier Roche Nature Reviews Chemistry (2020).(see publication)
2 The structures above (protein and small molecules) are presented for the sake of illustration only and are unrelated to the biological target and chemotypes of the project.

European Lead Factory https://www.europeanleadfactory.eu
The European Lead Factory (ELF) is a collaborative pan-European platform established to boost European drug discovery efforts. ELF’s 30 partners (large pharmaceutical companies, SMEs and Academic centers) work collaboratively towards building a 500,000-compound collection and associated biological screening facilities.
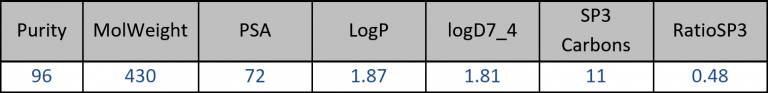
In this 5-year program, academic researchers from prestigious Universities and European CRO SME’s have joined their efforts to assemble a new screening collection of 200,000 compounds based on innovative scaffolds. Together with our academic partners, Profs Nelson and Marsden from the University of Leeds and Profs Clausen and Nielsen from the Technical University of Denmark , Edelris has set up a robust and efficient platform now able to steadily deliver a yearly average of 10,000 molecules and has achieved the objective of 40,000 sp3 rich compounds over 5 years.
The average physicochemical properties of our compounds are presented in the following table:
Edelris has published the following 11 articles in direct relationship with ELF
- Realisation of small molecule libraries based on frameworks distantly related to natural products
Anthony Aimon, George Karageorgis, Jacob Masters, Mark Dow, Philip G. E. Craven, Martin Ohsten, Anthony Willaume, Rémy Morgentin, Nicolas Ruiz-Llamas, Hugues Lemoine, Tuomo Kalliokoski, Andrew J. Eatherton, Daniel J. Foley, Stephen P. Marsden and Adam Nelson, Org. Biomol. Chem., 2018, Advance Article (see publication)
- Innovative approaches to the design and synthesis of small molecule libraries
A. Nelson, D. Roche, Bioorg. Med. Chem., 23, 2015, 2613 (see publication) - Design, synthesis and decoration of molecular scaffolds for exploitation in the production of alkaloid-like libraries
P. Craven, A. Aimon, M. Dow, R. Morgentin, N. Fleury-Brégeot, R. Guilleux, D. Roche, T. Kalliokoski, R. Foster, S.P. Marsden, A. Nelson, Bioorg. Med. Chem., 23, 2015, 2629-2635 (see publication) - Synthesis of 1,4,5 trisubstituted ɣ-lactams via a 3-component cascade reaction
M.A. Petersen, M.A. Mortensen, A.E. O’Hanlon Cohrt, R. Petersen, P. Wu, N. Fleury-Brégeot, R. Morgentin, C. Lardy, T.E. Nielsen, M.H. Clausen, Bioorg. Med. Chem., 23, 2015, 2695-2698 (see publication) - Aminomethylhydroxylation of alkenes: Exploitation in the synthesis of scaffolds for small molecule libraries
I. Colomer, O. Adeniji, G. M.Burslem, P. Craven, M. Ohsten Rasmussen, A. Willaume, T. Kalliokoski, R. Foster, S. P.Marsden, A. Nelson, Bioorg. Med. Chem., 23, 2015, 2736-2740 (see publication) - Synthesis of (Arylamido)pyrrolidinone Libraries through Ritter-Type Cascade Reactions of Dihydroxylactams
P. Wu, M. Åxman Petersen, R. Petersen, M. Ohsten Rasmussen, K. Bonnet, T. E. Nielsen, M. H. Clausen, Eur. J. Org. Chem., 2015, 5633-5639 (see publication) - Expansion of chemical space for collaborative lead generation and drug discovery – the European Lead Factory Perspective
A. Karawajczyk, F. Giordanetto, J. Benningshof, D. Hamza, T. Kalliokoski, K. Pouwer, R. Morgentin, A. Nelson, G. Müller, A. Piechot, D. Tzalis. Drug Discov Today, 2015, 1310-1316 (see publication) - Exploitation of the Ugi–Joullié reaction in the synthesis of libraries of drug-like bicyclic hydantoins
J.D. Firth, R. Zhang, R. Morgentin, R. Guilleux, T. Kalliokoski, S. Warriner, R. Foster, S.P. Marsden, A.Nelson. Synthesis, 2015, 2391-2406 (see publication) - Synthesis of sp3-rich Scaffolds for Molecular Libraries through Complexity-Generating Cascade Reactions
T. Flagstad, G. Min, K. Bonnet, R. Morgentin, D. Roche, M. H. Clausen, T. E. Nielsen, Org. Biomol. Chem., 2016, 4943-4946 - A metal-catalyzed enyne-cyclization step for the synthesis of bi- and tricyclic scaffolds amenable to molecular library production
P. Wu, M. Åxman Petersen, R. Petersen, A-E. Cohrt, R. Petersen, R. Morgentin, H. Lemoine, C. Roche, A. Willaume, M. H. Clausen, T. E. Nielsen, Org. Biomol. Chem., 2016, 6947-6950 (see publication) - Tandem Mannich/Diels-Alder reactions for the synthesis of indole compound libraries
P. Wu, M. Åxman Petersen, R. Petersen, T. Flagstad, R. Guilleux, M. Ohsten, R. Morgentin, T. E. Nielsen, M. H. Clausen, RCS Adv., 2016, 46654-46657
On behalf of Bayer HealthCare, a chemistry Hit-to-Lead optimization program was conducted by Edelris against ATAD2.
The epigenetic regulator ATAD2 has been proposed to play a key role in cancer control, however, further validation of the role of ATAD2 in different cancer indications was limited by the absence of selective, potent and cell active ATAD2 inhibitors.
We conducted the optimization of several chemical series of ATAD2 bromodomain binders initially identified from DNA-encoded compound libraries.
After applying a lean hit-to-lead campaign, our efforts resulted in the identification of BAY-850, a potent and cell-active isoform-selective ATAD2 chemical probe showing unprecedented chemical structure and mode of action.
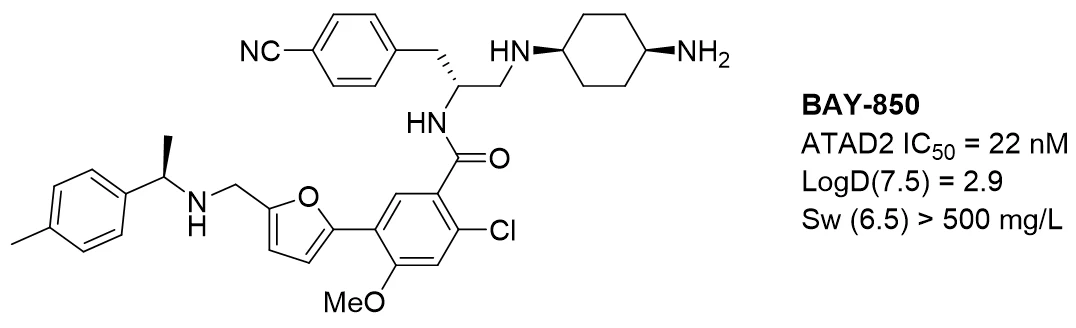
The work carried out by EDELRIS has improved the affinity, selectivity and diffusion in tissues.
Isoform-Selective ATAD2 Chemical Probe with Novel Chemical Structure and Unusual Mode of Action. DOI: 10.1021/acschembio.7b00708
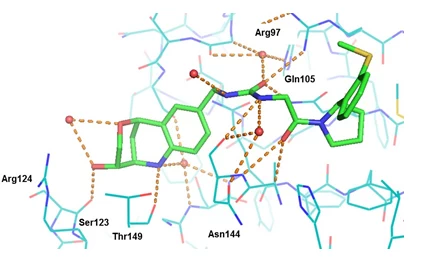
This case study, conducted on behalf of Galderma, aimed to identify novel inhibitors for the target Caspase-1. This enzyme is involved in various inflammatory diseases such as rheumatoid arthritis, osteoarthritis, or psoriasis.
Our drug design platform EDEN (Edelris Discovery ENgine) has been used for this objective, relying upon our proprietary database of natural product inspired fragments (Keymical Space™, 153,000 fragments). A scaffold hopping strategy was envisioned for scaffold replacement solutions using available structural information (X-ray structures of known inhibitors in complex with Caspase-1).
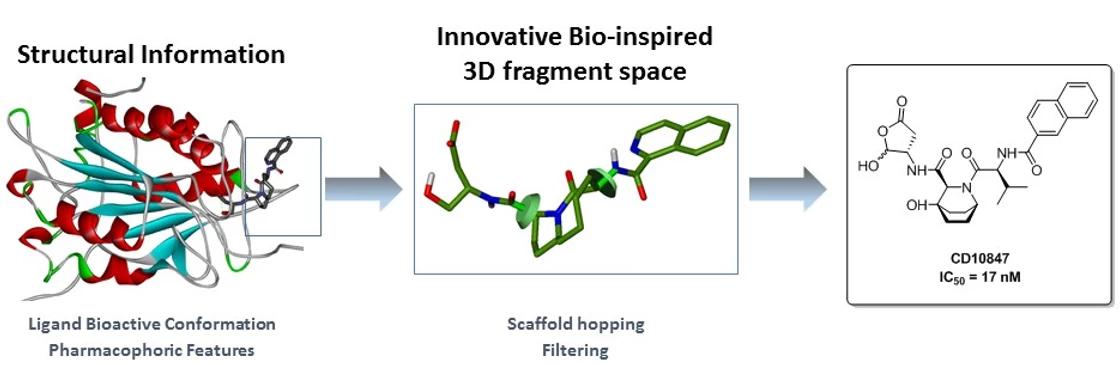
Within a few months, virtual hits could be converted by chemical synthesis into discrete compound libraries, ultimately leading to the novel inhibitor CD10847 (IC50 = 17 nM). Its predicted binding mode was experimentally confirmed by X-ray determination in the Caspase-1 protein. DOI: 10.1016
Peptidyl-prolyl isomerases (Cyclophilins) are extremely challenging targets. Although the druggability of this target class has been partly demonstrated in the 90s with Cyclosporin A (CsA), HTS campaigns failed to deliver valuable hits to identify small molecule inhibitors. Original, 3D-fragment hits from the Edelris Keymical Fragments™ collection were identified by EMD Serono using a fragment-based screening strategy. The fragment optimization program (FBLD) developed as a joint collaboration led to low molecular weight nanomolar inhibitors in two to three optimization cycles. View publication.